Cleanrooms are present in almost every industry to varying degrees. Maintaining a controlled environment is the gold standard way to create safe and quality products when considering precision manufacturing, research, and technology. Cleanrooms play a pivotal role in ensuring that sensitive processes are shielded from contaminants that could compromise the quality and integrity of products. However, not all cleanrooms are created equal, and industries vary in their requirements for cleanliness.
Understanding Cleanroom Classifications
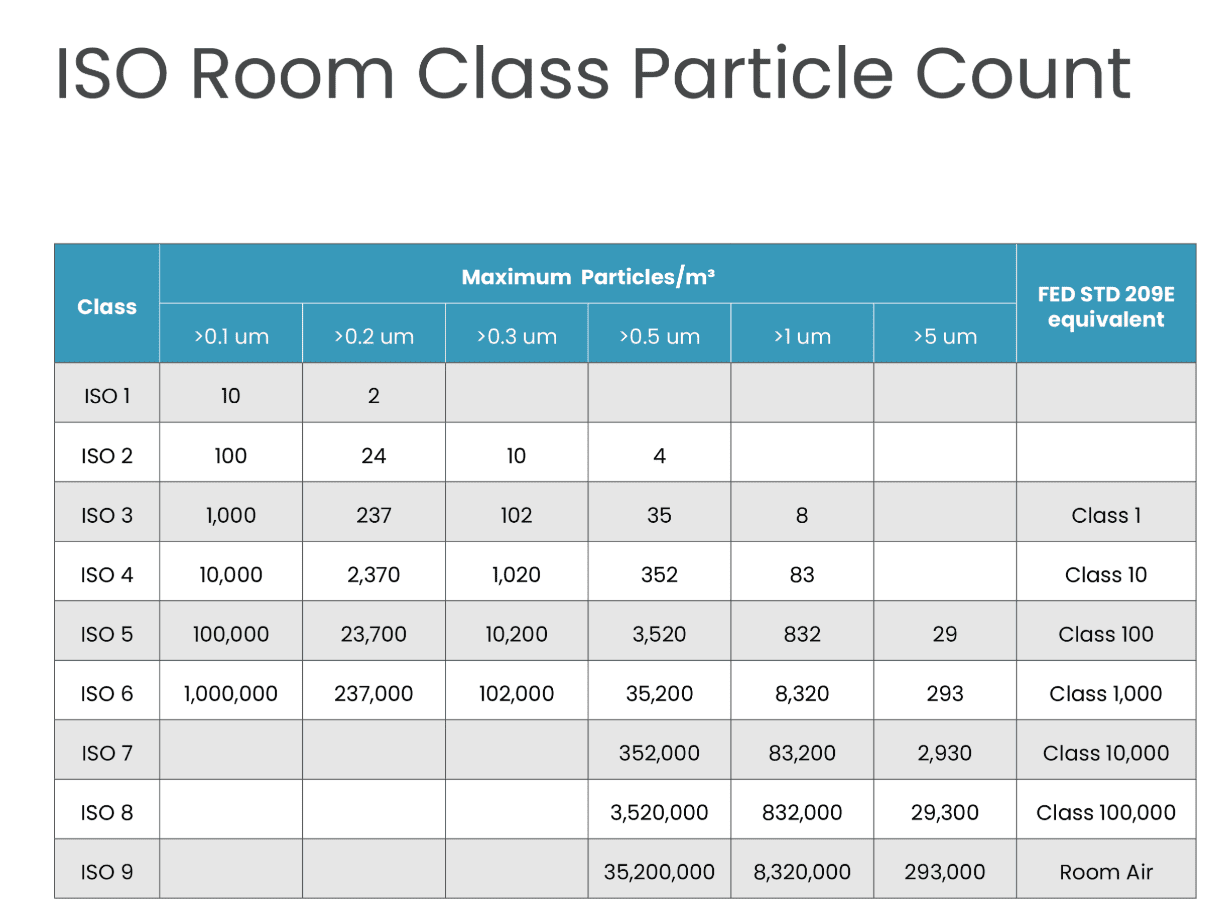
Cleanrooms are not one-size-fits-all. Cleanrooms are categorized based on the number of particles per cubic meter of air, as defined by the ISO (International Organization for Standardization). There are nine classifications: ISO 1, ISO 2, ISO 3, ISO 4, ISO 5, ISO 6, ISO 7, ISO 8 and ISO 9. ISO 1 is considered the cleanest, and ISO 9 is the dirtiest, or room air.
Cleanrooms, especially those adhering to Good Manufacturing Practice (GMP), are imperative for industries producing sterile medicinal products. Understanding the distinctions of cleanroom classification directly impacts compliance with federal and international standards like Annex 1 of the EU and PIC/S Guides to GMP.
ISO Clean Room Class Particle Count
Cleanroom standards are based on micrometres or microns. Every ISO level has a maximum limit for particles in each cubic meter of air (m³).
| Class | >0.1 um | >0.2 um | >0.3 um | >0.5 um | >1 um | >5 um | Federal Standard 209E Equivalent |
| ISO 1 | 10 | 2 | |||||
| ISO 2 | 100 | 24 | 10 | 4 | |||
| ISO 3 | 1,000 | 237 | 102 | 35 | 8 | Class 1 | |
| ISO 4 | 10,000 | 2,370 | 1,020 | 352 | 83 | Class 10 | |
| ISO 5 | 100,000 | 23,700 | 10,200 | 3,520 | 832 | 29 | Class 100 |
| ISO 6 | 1,000,000 | 237,000 | 102,000 | 35,200 | 8,320 | 293 | Class 1,000 |
| ISO 7 | 352,000 | 83,200 | 2,930 | Class 10,000 | |||
| ISO 8 | 3,520,000 | 832,000 | 29,300 | Class 100,000 | |||
| ISO 9 | 35,200,000 | 8,320,000 | 293,000 | Room Air |
Pharmaceuticals and Biotechnology
Cleanrooms in the pharmaceutical and biotechnology industries, classified as ISO 5 or ISO 6, are essential for producing medications, vaccines, and biological products. An ISO class 5 cleanroom, or a Class 100 cleanroom, with a maximum particle count of 3,520 particles per cubic meter, maintains a highly clean environment suitable for pharmaceutical processes. For sterile injectable drug production, an ISO classification 5 cleanroom minimizes the risk of particulate contamination, meeting stringent regulatory standards.
The pharmaceutical industry mandates the use of GMP cleanrooms to safeguard patient well-being. By adhering to these stringent guidelines, cleanrooms minimize the risk of contamination, ensuring the production of medicines that are not only effective but also free from harmful contaminants. Failure to comply with GMP standards could lead to the production of ineffective or even hazardous pharmaceuticals.
Electronics and Semiconductor Manufacturing
With some of the strictest rules, precision is the name of the game in electronics and semiconductor manufacturing, where cleanrooms typically fall within the ISO 2 to ISO 6 range. An ISO 3 cleanroom, or a Class 1 cleanroom, with a maximum particle count of 35,200 particles per cubic meter, is important for chip fabrication. An ISO 3 cleanroom environment prevents even invisible particles from compromising semiconductor device structures in microprocessor production. These industries also must combat electro-static discharge as another form of hazardous contamination that can damage equipment.
Aerospace and Defense
Controlled environments in the aerospace and defense industries usually range from ISO 5 to ISO 8. An ISO 7 cleanroom, with a maximum particle count of 352,000 particles per cubic. For satellite assembly, an ISO 5 cleanroom ensures foreign particles do not compromise the functionality of electronic components.
Dycem recently completed this GMP-compliant install for Space Park Leicester to protect their cleanrooms from unwanted particles that could contaminate products.
Medical Device Manufacturing
The production of medical devices demands cleanroom environments to ensure product safety and compliance. Cleanrooms classified as ISO 4 or ISO 5 are common in this industry, balancing the need for cleanliness with manufacturing efficiency. Manufacturing medical devices, especially those involving implants or devices that come into direct contact with the human body, have some of the most rigorous cleanroom standards to ensure product safety.
Medical device cleanrooms comply with Good Manufacturing Practice (GMP) regulations to assure patient health and safety. These regulations protect medical devices that come into contact with tissues and membranes and can impact patients’ well-being. GMP compliance is necessary to regulate consumables like medicine and devices designed to help with their consumption.
Medical devices are classified into three groups by FDA GMP regulations:
- Class 1: Low-risk things like bandages and tongue depressors that pose minimal harm to patients, where manufacturers must adhere to basic GMP outlines.
- Class 2: Medium-risk items like powered medical tools and infusion pumps, which have stricter quality control and documentation.
- Class 3: High-risk devices like implantable devices and life support equipment. These need the most rigorous safety checks, which could significantly impact a patient’s health.
Food and Beverage & Cannabis
While not as common as in other industries, food and beverage industry segments may adopt cleanroom standards for packaging processes, often using ISO 7 or ISO class 8 cleanrooms. In high-value food products or sensitive cannabis derivative production, an ISO 8 cleanroom prevents contaminants from compromising quality during packaging, ensuring product integrity.
The absence of standardized cleanroom practices in the cannabis industry has led many businesses to adopt best practices from other industries in their controlled environments. There has been great success in commercial cannabis cultivation in cleanroom environments by maintaining consistent and contaminant-free crop yields.
Cleanrooms vs. Controlled Environments
In industries without explicit cleanroom requirements, like cannabis, there is a growing trend to opt for controlled environments to enhance product quality and minimize contamination risks. Unlike traditional cleanrooms, controlled environments provide a regulated setting that ensures a level of control over factors such as air quality, temperature, and humidity.
This proactive approach reflects a commitment to maintaining the purity and integrity of products, aligning with the dedication to meet evolving consumer expectations for quality and safety. As regulations evolve and consumer awareness grows, the inclination towards implementing controlled environments is poised to become an integral facet of industries that may not have explicit cleanroom requirements, including sectors like personal care.
The imminent implementation of initiatives like MoCRA in the coming years further underscores the shift towards embracing controlled environments as a proactive measure to uphold product quality and address evolving regulatory standards.
Designing and Maintaining Cleanrooms
Building a cleanroom requires careful design and ongoing maintenance, and it is not a quick and easy process. Choosing construction materials that generate few particles and are easy to clean is essential to reduce contamination. The HVAC systems, acting as the lungs of the cleanroom, play an indispensable role in regulating and cleaning air quality.
Maintenance needs routine monitoring, like particle counts and airflow assessments, to ensure adherence to cleanliness standards. Aligning with industry-specific requirements and emphasizing practical design and maintenance practices ensures that cleanrooms consistently meet regulatory demands, safeguarding the integrity of processes and products.
Future Trends in Cleanroom Technology
As technology advances, cleanroom technology evolves to meet new challenges:
- The latest trends in cleanroom design include modular construction, where prefabricated sections are assembled on-site, leading to faster construction, cost reduction, and enhanced design flexibility.
- There is a growing reliance on robotics and automation for various cleanroom tasks, improving efficiency, reducing contamination risks, and allowing human workers to focus on more intricate responsibilities.
- Integrating Internet of Things (IoT) devices and smart sensors in cleanrooms. These technologies gather real-time temperature, humidity, and particle count data, enabling proactive monitoring and troubleshooting to prevent contamination.
Cleanroom Solutions with Dycem
Cleanroom classifications serve as a foundation for industries striving to maintain high quality, safety, and precision standards. By understanding the specific cleanroom requirements of each industry, companies can create controlled environments that meet regulatory standards and optimize production processes. In the ever-evolving landscape of technology and manufacturing, adapting cleanroom classifications to industry needs is essential for the success of future product development.
Whatever industry you’re in, Dycem has the contamination control mat solution for you. 80% of contaminants enter a cleanroom or controlled environment at the floor level via shoes or wheels. At Dycem, our mission is to provide solutions to minimize this risk effectively and efficiently.
Shop our solutions and contact us today with any questions.