What are medical devices?
A medical device is any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material, or other similar or related article, intended by the manufacturer to be used, alone or in combination for a medical purpose (World Health Organisation). This can include syringes, bandages, blood glucose meters, and catheters.
How big is the medical device industry and why has demand increased?
Driven by factors such as the growing prevalence of chronic disorders, the rise of the geriatric population and a rise in infectious diseases, the global value of the medical device industry is expected to grow from $495.46 billion in 2022 to $718.92 billion in 2029 (Fortune Business Insights).
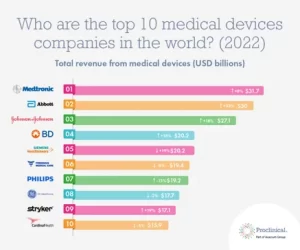
Last year, Proclinical released a list of the ‘Top 10 medical device industries in the world’, with companies like Medtronic and Abbott topping the list.
How is the production of medical devices regulated?
The FDA’s Center for Devices and Radiological Health (CDRH) for the US or The Medicines and Healthcare products Regulatory Agency (MHRA) in the UK are responsible for regulating firms that manufacture, repackage, relabel, and/or import medical devices.
Medical devices are classified based on risk and into Class I, II, and III. The higher the class, the higher the regulatory control. Class I is regarded as low-risk and includes devices such as wheelchairs, stethoscopes, and spectacles. Class II is regarded as medium risk and includes devices such as dental fillings, surgical clamps, and lung ventilators. Class III is regarded as high-risk and includes devices like pacemakers, heart valves, and implanted cerebral stimulators (MHRA).
Why is contamination control important when manufacturing medical devices?
Contamination poses a huge risk to the medical device manufacturing industry. Contaminated devices can lead to product recalls, plant shutdowns, and health & safety issues. This is especially true for the high-risk medical devices which are often introduced into sterile body areas. If these devices are contaminated the outcome could be devastating and may lead to possible infections.
In 2016, a medical device manufacturer voluntarily recalled a batch of syringes due to possible Burkholderia cepacia (B. cepacia) contamination. The Centers for Disease Control and Prevention (CDC) identified the contaminated syringes as a source of B. cepacia infections outbreaks in various regions of the US, and caused 163 people to be affected, including 7 deaths.
Due to the stringent measures of production, medical devices are usually manufactured in cleanrooms ranging from ISO 5 to ISO 8. The higher the risk and more complex the medical device, the higher level of cleanroom classification.
It is important that all cleanrooms have a contamination control strategy in place, involving airflow control, environmental monitoring, personnel regulations, gowning practices, and specific cleaning and sanitisation protocols. But do they focus on floor-level protection?
How can Dycem help?
Dycem Contamination Control flooring and mats capture and retain up to 99.9% of contamination from feet and wheels, and 75% of airborne contamination, preventing it from entering cleanrooms.
We help to protect cleanrooms from the outside, in. Surrounding cleanrooms are layers of other areas leading to the middle which is the cleanest and most critical environment. Placing Dycem outside of this critical, middle area helps to remove contamination from the soles of shoes and wheels as they pass through each layer of the surrounding environment.
Dycem is to be used in conjunction with other contamination control protocols to give your facility the highest level of protection. Our mats and flooring are long-lasting, with a lifespan of 3-5 years, washable, and have antimicrobial properties.
